Abstract
Recent advances in CRISPR/Cas9 technology allowing precise genome editing at a site of interest have enabled relevant human disease modeling and the development of corrective gene therapies for various genetic disorders. Paroxysmal nocturnal hemoglobinuria (PNH) is a hematological disorder linked to acquired somatic loss-of-function mutations disrupting the X-linked PIG-A gene in hematopoietic stem and progenitor cells (HSPC), characterized by the clinical triad of intravascular hemolysis, thrombosis and bone marrow failure, as well as clonal expansion of HSPC defective in glycosylphophatidylinositol(GPI)-linked proteins, due to loss of PIG-A enzymatic activity. There have been many attempts to model PNH utilizing conditional knockout strategies in mice, but these do not recapitulate the hemolytic phenotype or clonal dominance. Whether clonal expansion is intrinsic to PIG-A deficient HSPC or extrinsic, resulting from relative protection of PIG-A deficient HSPC and progeny cells from immune attack, is unclear. To explore the pathophysiologic mechanisms of PNH HSPC clonal expansion, we generated a relevant non-human primate model for PNH, utilizing autologous transplantation of HSPC edited with CRISPR/Cas9 at the PIG-A locus.
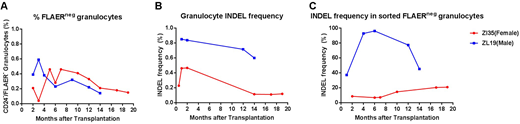
The most efficient guide RNAs (gRNAs) targeting several sites within PIG-A exon 2 locus on the X chromosome, where most of mutations occur in patients with PNH, were selected based on efficiency of editing as screened in FRhK-4 cell lines and mobilized macaque CD34+ HSPC. Compared to editing of the control AAVS1 locus and other genes of interest, editing of all PIG-A locus sites was relatively inefficient. Autologous HSPC were transduced with ribonucleotide protein (RNP) complexes of Cas9 protein and selected gRNAs targeting either PIG-A or the AAVS1 "safe harbor" site, as an internal control, followed by transplantation into one female (ZI35) and one male (ZL19) macaque, respectively. Both animals engrafted promptly and clones acquiring the PNH loss-of-function phenotype, defined by loss of binding of FLAER, a fluorescent compound that binds to all GPI anchors, and loss of expression of lineage-specific GPI-linked proteins (CD24 for granulocytes and CD14 for monocytes) were stably maintained at levels of 0.2-0.4% for up to 19 months post-transplantation, with no evidence for intrinsic clonal expansion of PIG-A edited cells (Fig. 1A). Upon targeted deep sequencing, 0.4-1% insertions and deletions (INDELs) induced by CRISPR/Cas9 were detected, and the predominant INDEL type was identified as a single base deletion at the +1 or -1 positions of the target site consistently in granulocytes and other mature hematopoietic lineage cells from peripheral blood (PB), suggesting that initial mutations occurred in long-lasting HSPCs rather than short-term progenitor cells. Furthermore, as the PIG-A gene is located on X chromosome, we sought to investigate the difference in genome editing efficiency depending on the number of X alleles or activation state. Note that the expected INDEL frequency in completely PIG-A deficient sorted FLAERneg cells would be 100%, however, large deletions or rearrangements are not detected by standard deep sequencing methodologies. Interestingly, the mutation frequencies in total granulocytes and more importantly in sorted FLAER negative PNH cells were always much higher in male (ZL19) macaque cells than in female (ZI35) macaque cells (Fig. 1B and C). Consistently, gene editing PIG-A allele efficiency with CRISPR/Cas9 was also higher in human male B-lymphoblastoid cell lines (LCL) compared to female cells, 23.2% versus 16.6% (n=5), respectively. The finding that edited allele frequency was consistently lower in sorted FLAERneg female than male cells suggests that editing of the active X allele may be favored, potentially due to poor accessibility of inactive loci to editing machinery.
In conclusion, we have successfully established a rhesus macaque model for PNH utilizing autologous transplantation of CRISPR/Cas9 edited HSPC. To date, we found no evidence for intrinsic expansion of PIG-A deficiency HSPC and hematopoietic progeny. This modeling approach could be utilized for further investigation of extrinsic or intrinsic factor responsible for clonal expansion. Furthermore, our findings provide a better insight into the relationship between CRISPR/Cas9 editing efficiency and active versus inactive X-linked genes.
Dunbar:National Institute of Health: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal